High Temperature Oxidation
From Thermogravimetry to in-situ Oxidation – Mechanistic Investigation on Oxide Layer Formation at High Temperatures
In the frame of two DFG-funded projects we are studying mechanisms of high temperature oxidation of superalloys for over 10 years. These research projects are a continuation of a long tradition of earlier work at the Chair of Surface Science and Corrosion. While the earlier research was mainly concerned with high temperature corrosion under aggressive environments, the current research activities are focused on the growth of oxide layers in synthetic air or in pure oxygen. The goal of our research is to contribute to the understanding of elementary mechanisms of oxide formation on traditional and novel alloys for high temperature applications. Of special interest are Ni- and Co-base superalloys. The research is carried out with a variety of experimental approaches.
Thermogravimetry
Classic, isothermal oxidation experiments typically are mainly concerned with the detection of mass gain of a sample during oxide growth. This gives information on the kinetics of oxidation reactions and hence on the oxide growth mechanisms. The equipment used for thermogravimetry at our institute is able to detect small mass gains, and in addition experiments can be carried out under controlled atmospheres. After the oxidation experiments the samples are characterized with high-resolution surface analytical techniques (e.g., AES, ToF-SIMS).
Another important information is the time-dependent growth of mostly complex, multilayered oxide scales. Here the focus is on the formation of protective oxide layers (e.g., Al2O3, Cr2O3). For efficient investigations of a number of samples for different oxidation times, an additional vertical oven can be used to oxidize many samples under controlled atmosphere.
In-situ oxidation experiments in a scanning electron microscope
Using a special heating stage it is possible to oxide samples at low pressures in an electron microscope. Changes in surface morphology during the experiments can be documented with a high resolution. For instance, for polycrystalline materials grain boundaries play a key role in formation and growth of oxide layers. In addition, microstructure or grain orientation can significantly influence the propagation of oxidation (see Figure).
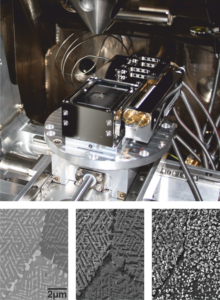
This method is especially well suited to study the influence of grain orientation on the initiation stages of oxide formation. As multiple grains with varying orientations are present on the sample surface next to each other, one can gain a multitude of information from each sample. The oxygen availability in the vicinity of the sample can be kept on a relatively low level. Hence, even very small influences on the critical processes, such as nucleation of oxides, can be visible.