Materials in Medicine
Magnesium-based materials for biodegradable implants
Magnesium is being considered as a suitable material for applications in biodegradable implants, both in the fields of stenting and bone fixation.
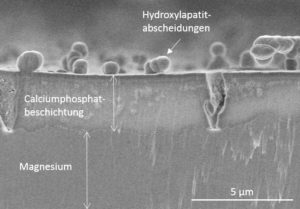
Degradation in the body is due to corrosion of Mg in the physiological environment. The influencing factors are the body temperature (37 °C), buffered pH value of ca. 7.4, high chloride-concentration, and flow. Moreover, organics components of body fluids as well as cells are influencing Mg corrosion. Pure Mg shows an initially high corrosion rate, combined with strong hydrogen gas evolution and local alkalization. To minimize these undesired effects, research is searching for suitable alloys with optimized properties. Another approach to decrease the initially high corrosion rate is coating of Mg.
In our group, we carry out electrochemical and surface analytical studies on different Mg alloys in view of their short- and long-term degradation behavior. To complete the corrosion investigations, electrochemical impedance spectroscopy and polarization curves are used in combination with H2-gas evolution and weight loss measurements. Moreover, we are developing novel coatings to slow down Mg corrosion, to functionalize the surface, or to increase the bioactivity of Mg surface.
Ti-based implant materials

Titanium and its alloys belong to the most corrosion resistant and biocompatible materials in the field of implantology and orthopedics. Spontaneous formation of a very stable TiO2 passive film on the alloy surface and a relatively low modulus of the materials lead to an optimized interactions between the implant and bone. Due to the high biocompatibility of Ti, no toxic reactions take place, leading to fast healing. In spite of these already very good properties of Ti-based materials, the biocompatibility and the implant/bone-interactions can be further significantly enhanced by nanometer-scale surface modification. Human bone is hierarchically built from nanometer- to micro- and to macroscale, and hence nanostructuring the surface can play a key role for osseointegration, but also for protein adsorption and cell adhesion on surfaces.
Our research deals with a variety of nanostructured surfaces of a TiAl6V4 alloy in view of corrosion resistance, biocompatibility and bioactivity. Electrochemical measurements and immersion testing combined with surface characterization with a variety of analytical tools such as SEM, XPS, ToF-SIMS, XRD and FTIR are carried out to determine the influence of the nanostructured coatings on the surface performance. Immersion experiments are carried out in an incubator, to simulate in vivo conditions with temperature of 37.5 °C and CO2-content of 5 %.
Stainless steel for biomaterials
Austenitic stainless steel 316L is widely used in the biomaterials field. Between some applications are orthopedic fracture plates, artificial knee, hip joints and dental implants. Stainless steel is a cheaper alternative to Ti alloys used for these implants. In this institute, self-assembled monolayers and hydrophobic coatings are used to change the cytocompatibility, it also leads to a decrease of the bacterial adhesion in biomaterials exposed to protein solutions.